Abstract
Introduction:
Cerebral blood flow (CBF) is increased in sickle cell disease (SCD) to compensate for chronic hemolytic anemia. Since the brain is strongly dependent on adequate oxygen levels, severe anemia may result in ischemia and silent cerebral infarctions (SCIs), which are present in the majority of patients with SCD. Besides CBF, the cerebral metabolic rate of oxygen (CMRO2), representing the cerebral oxygen consumption, can be measured using specialized MRI techniques. CMRO2 is dependent on CBF, OEF oxygen extraction fraction (OEF) and the hematocrit in the cerebral circulation. In order to maintain stable CMRO2 the OEF changes to compensate for fluctuations in CBF. Previous studies found either elevated or reduced rather than stable CMRO2 in SCD. To further explore the regulation of cerebral oxygenation in SCD, we measured CMRO2, OEF and CBF using MRI at rest and upon administration of acetazolamide (ACZ), a non-metabolic vasodilator. In addition, we related the CMRO2 to the prevalence and location of SCIs and to laboratory parameters of hemolysis.
Methods:
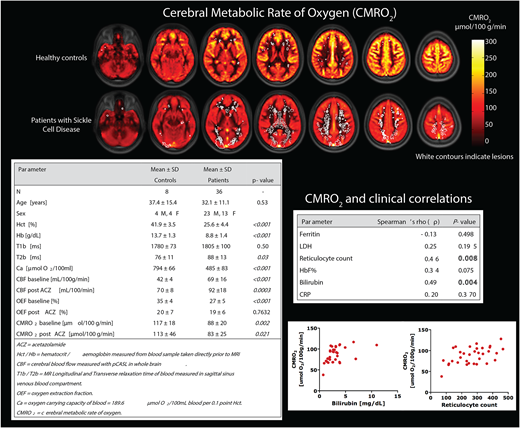
Adult SCD patients (HbSS/HbSβ0) without a history of stroke, and healthy age-, sex, and race-matched controls were recruited for this IRB-approved MRI study with ACZ-induced vasodilation and venous blood sampling. MRI images were acquired at 3T (Philips Healthcare, Best, NL). Sequences included 3D FLAIR with 1mm isotropic resolution for lesion evaluation, longitudinal and transverse relaxation times of blood (T1b and T2b) in the cerebral sagittal sinus using a T2 prepared tissue relaxation with inversion recovery sequence (T2-TRIR), and pseudo-continuous arterial spin labeling (ASL) for whole brain CBF measurements. T1b was used to correct ASL-based CBF values. T2b was converted to venous saturation (Yv) using a recently published SCD-specific model, which was calibrated in sickle blood rather than bovine blood. CBF was measured at baseline and 10 min post acetazolamide (ACZ) (16mg/kg intravenous infusion over 3min). Images were co-registered and quantified using the ExploreASL toolbox.
CMRO2 was calculated as follows: CMRO2 = CBF * OEF * Ca, where CBF is the whole brain CBF map from ASL, OEF is the arteriovenous oxygen saturation (Y) difference (Ya-Yv/Ya) derived from T2 measurements, and Ca is the oxygen carrying capacity of blood calculated from hematocrit sampled immediately prior to MRI. Blood markers of hemolysis (reticulocyte count and bilirubin) were correlated to MRI hemodynamic markers using bivariate Spearman's rho (ρ). Group comparisons were tested using Student's t-tests. P values <0.05 were considered statistically significant.
Results:
As expected, CBF was significantly higher in patients with SCD (69 ± 16 mL/100g/min) compared to healthy controls (42 ± 4 mL/100g/min, P <0.001), whereas oxygen carrying capacity (Ca) was significantly lower in patients with SCD vs healthy controls due to lower hematocrit (485 ± 83 vs 794 ± 66 μmol O2/100ml blood, P=0.001). At baseline, mean CMRO2 was lower in patients with SCD compared to healthy controls (88 ± 20 vs 117 ± 18 μmol/100 g/min, P = 0.002), contrary to our hypothesis, indicating a reduced oxygen metabolism in patients with SCD. After acetazolamide, CMRO2 remained the same due to an increase in CBF (P<0.001) and a compensatory reduction in OEF. The reduction in OEF in patients with SCD and healthy controls (20 ± 7 vs 19 ± 6, respectively P= 0.76) indicates that OEF adjusts appropriately according to the increased flow. By mapping CMRO2 and the locations of SCIs, we found that lesions were most prevalent in the regions with the lowest CMRO2 corresponding to the deep white matter and borderzone regions, supporting an ischemic etiology of lesions. High hemolytic rate was associated with higher CMRO2 most likely due to an increased CBF.
Conclusion:
We observed reduced CMRO2 in patients with SCD compared to healthy controls due to low OEF. A reduced CMRO2 could pose a risk for ischemia, despite high flow rate delivering oxygen, because of low OEF. This is supported by the fact that the silent cerebral infarcts are located in regions with the lowest CMRO2. We postulate that patients with SCD have a reduced capacity to increase the OEF in regions with inadequate CBF resulting in local ischemia and local infarction. The pathogenesis of the reduced OEF remains unclear but could be related to arteriovenous shunting whereby there is insufficient time for oxygen to dissociate.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal